E hana i nā mole o ka nā mea hoʻohui kemika, pono e hui pū nā ʻĀtoma o nā mea ʻokoʻa a i ʻole nā mea ʻē aʻe i kekahi i kahi ala paʻa, a hiki i kēia ke hana ma nā ʻano like ʻole e ka mana o nā hanana hanana i loaʻa i kēlā me kēia atoma, a, e like me kā mākou e ʻike nei, he nuklei i hoʻopiʻi maikaʻi ʻia e hoʻopuni ʻia e ke ao uila.
Hoʻonohonoho maikaʻi ʻole ʻia nā uila uila a noho kokoke i ka nucleus no ka mea ka ikaika electromagnetic ʻumeʻume iā lākou. ʻO ka pili o ka electron i ka nucleus, ʻo ka nui o ka ikehu e pono ai e hoʻokuʻu iā ia.
Akā ʻaʻole like nā mea āpau: loaʻa i kekahi mau ʻano e nalo i nā electrons o waho o ke ao (nā mea me ka ikehu ionization haʻahaʻa), ʻoiai e hopu ana kekahi iā lākou (nā mea me ka pili o ka electron kiʻekiʻe). Hana kēia no ka mea e like me ka lula ʻo octet Lewis, pili ke kūpaʻa me ke kū ʻana o 8 mau electron i ka iwi waho loa a i ʻole orbital, ma ka liʻiliʻi i ka nui o nā hihia.
A laila pehea malia paha e lilo a loaʻa paha nā uila, hiki ke hana ʻia nā ion o ka ʻaoʻao ʻē aʻe, a ʻo ka hoʻohālua electrostatic ma waena o nā iona o ka ʻaoʻao ʻē aʻe e hoʻohui a hana i nā mea hoʻohui kemika maʻalahi, kahi i hāʻawi ai kekahi o nā mea i nā uila a me nā mea ʻē aʻe i loaʻa iā lākou. I hiki i kēia ke hana a a hoʻopaʻa lono pono ia aia he ʻokoʻa a i ʻole delta o ka electronegativity ma waena o nā mea e pili ana ma ka liʻiliʻi 1.7.
ʻO ka hoʻopaʻa lono Hana ʻia ma waena o kahi hui metallic a me kahi metallic ʻole: hāʻawi ka ʻāpana metala i hoʻokahi a ʻoi aku mau uila uila a no laila hana i nā ion i hoʻoili pono ʻia, a loaʻa nā nonmetal iā lākou a lilo i ʻāpana i hoʻopiʻi ʻia (anion). ʻO nā metala alkali a me nā alkaline honua nā mea e hana i nā cations ʻoi loa, a ʻo nā halogens a me oxygen ka mau anion.
Laulaha, nā mea hoʻohui i hana ʻia e nā paʻa ionic he nā paʻa ma ka mahana o ka lumi a me ke kiko hoʻoheheʻe kiʻekiʻe, hiki ke hoʻoheheʻe ʻia i ka wai. I ka hopena he nui lākou nā alakaʻi maikaʻi o ka uilaʻoiai he mau electrolytes ikaika lākou. ʻO ka ikehu lattice o kahi ionic solid ka mea e māka ai ka ikaika uʻi ma waena o nā ʻākona o kēlā paʻa.
Hiki iā ia ke lawelawe iā ʻoe:
- Nā laʻana o nā Bond Covalent
- ʻĀpana magnesium (MgO)
- Sulphate keleawe (CuSO4)
- Potassium iodide (KI)
- Zinc hydroxide (Zn (OH) 2)
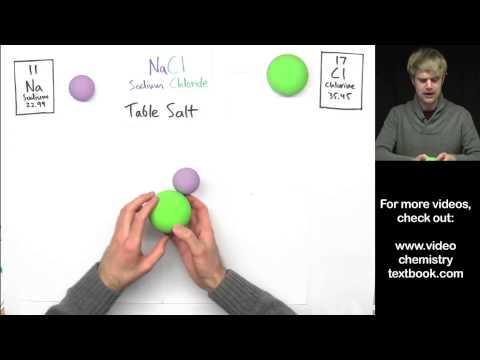
- ʻO Sodium kloride (NaCl)
- Nitrate kālā (AgNO3)
- Pāpaʻi lika (LiF)
- Makanekiuma koloriside (MClCl2)
- Pāpūkona hydroxide (KOH)
- ʻO ka nitrate kalipuna (Ca (NO3) 2)
- Pākuʻi phosphate (Ca3 (PO4) 2)
- Potassium dichromate (K2Cr2O7)
- Disodium phosphate (Na2HPO4)
- Pipi sulfide (Fe2S3)
- ʻO ka potassium bromide (KBr)
- Kalepona kalapona (CaCO3)
- ʻO Sodoma hypochlorite (NaClO)
- ʻO ka potassium sulfate (K2SO4)
- Manganese chloride (MnCl2)