Anter
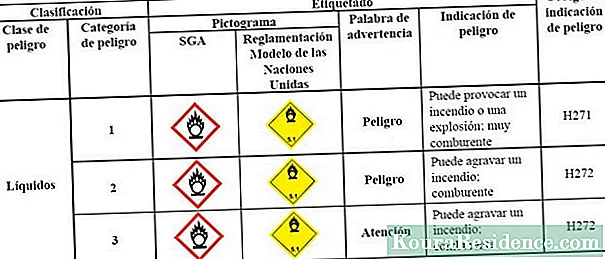
Nā mea mea hoʻoheheʻe (O) nā mea hoʻoheheʻe ʻia, ma lalo o nā kūlana kikoʻī o ka mahana a me ke kaomi, hiki ke hui pū me kahi wahie a hana, pololei, i hoʻā ʻana. I kēia kaʻina hana e hoʻoliʻiliʻi ka mea hoʻowali i ka wahie a ʻo ka mea hope i oxidized e ka mea mua.
ʻO nā oxidizers nā ʻokena oxidizing, prone i ka exothermic hoʻemi-oxidation i nā hopena (hana lākou i ka wela), no laila he nui nā ʻano o nā mea i manaʻo ʻia i waena o nā mea weliweli a i ʻole ka mālama pono ʻana, ʻoiai hiki iā lākou ke hana i nā wela nui.
Kapa ʻia ʻo oxidizer, ma o ka hoʻolōʻihi ʻana, i nā mea waena i hiki ai ke kuni.
E nānā pū: Nā laʻana o nā wahie
"Redox"
ʻO ka mea hoʻoheheʻeMa ke ʻano he oxidants, hana lākou i nā hopena ʻo "redox", ʻo ia hoʻi, ka hoʻohaʻahaʻa like a me ka hoʻowali ʻana. I kēia ʻano hopena, hiki ke hoʻololi i ka electron i ka nui o ka loaʻa o ka oxidant electrons (hoʻoliʻiliʻi) a lilo ka mea reducer i mau electrons (oxidizes). Hoʻopili nā mea āpau āpau, me ka hoʻohui.
ʻO nā laʻana o kēia ʻano ʻano nā hopena o ka pahū, synthes kemika a me ka popopo.
Nā laʻana o nā oxidizers
- Oxygen (ʻO2). ʻO ka maikaʻi o ka oxidizer, pili i ka hopena o nā hopena flammable a pahū paha. ʻO ka ʻoiaʻiʻo, ʻaʻole hiki i ke ahi maʻamau ke hana i kona wā e hele ʻole ai. Ma ka laulā, hana nā hana redox mai ka oxygen e hana, i ka hoʻohui i ka ikehu, nā nui o CO2 a me ka wai.
- Ozone (ʻ.3). ʻO kahi mole kinoea i ʻike ʻole ʻia i ke kaiapuni, ʻoiai ʻoi aku ka nui i nā papa o luna o ka lewa, hoʻohana pinepine ʻia ia i ka hoʻomaʻemaʻe wai a me nā hana ʻē aʻe e hoʻohana ana i ka hiki ke hana i ka hana oxidizing ikaika.
- Hydrogen peroxide (H2A I OLE2). ʻIke ʻia hoʻi me he hydrogen peroxide a i ʻole dioxogen, he polar nui ia, wai hoʻoheheʻe ʻia, hoʻohana pinepine ʻia e hōʻino i nā ʻeha a i ʻole lauoho huluhulu. Kūleʻa ʻole kāna ʻano a mālama ʻia e wāwahi i loko o ka wai a me nā mole oxygen, e hoʻokuʻu ana i ka ikehu wela i ke kaʻina. ʻAʻole hiki ke wela, akā hiki iā ia ke hoʻoulu i ka puhi kūlohelohe i ka wā o ke keleawe, kālā, keleawe a i ʻole kekahi meaola..
- Hypochlorites (ClO-). Hoʻopili ʻia kēia mau ʻona i loko o nā pūhui like ʻole e like me ka wai bleach (sodium hypochlorite) a i ʻole nā pauka (calcium hypochlorite), kūpaʻa ʻole a makemake e decompose i ke alo o ka lā, ka wela a me nā kaʻina hana ʻē aʻe. Hana lākou i ka exothermically loa i nā meaola, hiki ke kumu i ke ahi, a me ka manganese, e hana ana i nā permanganates..
- ʻO Permanganates. ʻO kēia nā paʻakai i loaʻa mai ka permanganésic acid (HMnO4), kahi a lākou e hoʻoilina ai ka anion MnO4– a no laila manganese i loko o kāna kūlana ʻoi loa. Kūleʻa lākou i kahi kala viola ikaika a me kahi flammability kiʻekiʻe loa i ka pili ʻana me nā meaola., ke hana nei i kahi lapalapa ahi a hiki ke hana i nā wela nui.
- ʻAkika peroxosulfuric (H2SW5). ʻO kēia paʻa paʻa ʻole waihoʻoluʻu, hoʻoheheʻe ʻia ma 45 ° C, he nui nā ʻoihana ʻoihana he disinfectant a hoʻomaʻemaʻe, a i ka hanauna o nā paʻakai waikawa i ke alo o nā mea e like me ka potassium (K). I ke alo o nā moleolaola, e like me nā ether a me nā ketones, hana ʻia nā mole kūpaʻa ʻole ma o ka peroxygenation, e like me ka acetone peroxide.
- Acetone peroxide (C9H18A I OLE6). ʻIke ʻia he peroxyketone, he mea pahū nui kēia hui meaola, no ka mea maʻalahi ka wela, ka hakakā a me ka hopena. ʻO ia ke kumu i hoʻohana ai nā mea hoʻoweliweli he detonator i kā lākou hoʻouka kaua ʻana a ʻaʻole i hōʻeha ʻia kekahi mau kemika i ka lawelawe ʻana iā ia. He mole kūpaʻa paʻa ʻole ia, a i ka wā e decomposed i loko o nā mea paʻa hou aʻe hoʻokuʻu i ka nui loa o ka ikehu (entropic pahū).
- ʻO Halogens. ʻO kekahi mau mea o ka pūʻulu VII o ka papa manawa, i ʻike ʻia ʻo halogens, makemake e hana i nā iona mononegative ma muli o ko lākou pono no nā uila e hoʻopau i kā lākou pae ikehu hope loa. pēlā e hana ai i nā paʻakai i ʻike ʻia me nā halide e hoʻohaʻahaʻa nui ʻia.
- Mea hoʻohoihoi ʻo Tollens. Ua kapa ʻia e ka mea kelemania Kelemania ʻo Bernhard Tollens, kahi wai aineous o ka diamine (ʻelua mau amine: NH3) a me ke kālā, o ka hoʻohana hoʻokolohua i ka ʻike ʻana i nā aldehydes, ʻoiai ka hiki i kā lākou hiki ke hana oxidizing hiki ke hoʻolilo iā lākou i mau waikawa carboxylic. Eia nō naʻe ʻo Tollens reagent, inā mālama ʻia no ka manawa lōʻihi, hana wale ia ka fulminate kālā (AgCNO), kahi paʻakai kālā pahū nui..
- ʻO Osmium Tetroxide(Keawe4). ʻOiai ka rarity o osmium, loaʻa i kēia hui he mau noi hoihoi, hoʻohana, a me nā waiwai. I ka paʻa, no ka laʻana, he maʻalahi loa ia: lilo ia i gas i ke ana wela o ka lumi. ʻOiai he oxidant mana, me nā hoʻohana he nui i ke keʻena hoʻokolohua ma ke ʻano he catalyst, ʻaʻole ia e pane me nā mea ʻaihue, akā he ʻawahia nui ia i ka nui o nā mea i hiki ke ʻike ʻia e ka honi kanaka.
- ʻO nā paʻakai Perchloric acid (HClO4). Nā paʻakai Perchlorate i loko o ka chlorine i kahi mokuʻokikena kiʻekiʻe, e kūpono ana iā lākou no ka hoʻohui ʻana i nā mea pāhū, nā pono hana pyrotechnic a me nā wahie rocket, no ka mea he oxidizer nui lākou me ka liʻiliʻi liʻiliʻi.
- Nā Nitrates (NO3–). E like me nā permanganate, he paʻakai lākou kahi kahi a ka nitrogen e noho ai i kahi mokuʻāina ʻoi loa. Hōʻike kūlohelohe kēia ʻano hui i ka palaho ʻana o nā ʻōpala olaola e like me ka urea a i ʻole kekahi mau protein nitrogenous, e hana ana i ka amonia a i ʻole amonia, a hoʻohana ākea ʻia i nā mea momona. He ʻāpana nui ia o ka pauka ʻeleʻele, e hoʻohana ana i kona mana hoʻoheheʻe e hoʻololi i ke kalapona a me ka luaipele a hoʻokuʻu i ka ikehu caloric..
- Nā Suloxides. Loaʻa ʻia e ka oxidation organik o sulfides, hoʻohana ʻia kēia ʻano hui i nā lāʻau lapaʻau lehulehu a i ke alo o ka nui o oxygen e hiki iā lākou ke hoʻomau i kā lākou kaʻina hana oxidation a lilo i mau sulfones, pono e like me antibiotics.
- ʻO Chromium trioxide (CrO3). ʻO kēia hui kahi paʻa o ka ʻulaʻula ʻulaʻula, soluble i ka wai a pono i nā kaʻina hana o ka galvanizing a me ka chromating o nā metala. ʻO ka launa wale ʻana me ka ethanol a i ʻole nā mea ʻokanika ʻē aʻe e kumu i ka hoʻā koke ʻana o kēia lāʻau., ka mea hoʻowahāwahā nui, ʻona a me ka carcinogenic, a me kahi ʻāpana nui o ka hexavalent chromium, kahi hui weliweli loa no ke kaiapuni.
- Pūhui me cerium VI. ʻO Cerium (Ce) kahi mea kemika o ke kauoha o nā lanthanides, kahi mea kila palupalu hina, ductile, oxidized maʻalahi. Hoʻohana ākea ʻia nā cerium oxides ʻokoʻa i nā ʻoihana, keu hoʻi i ka hana ʻana i nā match a ma ke ʻano he pōhaku māmā ("tinder") ma o kahi mea hao me ka hao., ʻoiai ʻo ka hakakā wale nō me nā ʻilikai ʻē aʻe ua lawa ia e hana i nā huna ahi a me ka wela hiki ke hoʻohana ʻia.
Hiki iā ia ke lawelawe iā ʻoe:
- Nā laʻana o nā ʻAilika i ke ola o kēlā me kēia lā